
Dr. Matthew Wheater
Consultant Medical Oncologist, University Hospital Southampton
Dr Wheater provides an overview of the FOCUS study, which included 91 metastatic uveal melanoma patients. Dr Wheater explains the study design, patient selection and study outcomes.
The FOCUS study was the largest LDT (liver directed therapy) clinical trial undertaken in metastatic uveal melanoma.1-3

95 Patients were enrolled into the CHEMOSAT arm; 91 patients received treatment.4,a

Patients could have limited extrahepatic disease in the bone, subcutaneous sites, lymph nodes, or lung if the life-threatening component of the UM was in the liver. Any extrahepatic disease needed to be amenable to resection or radiation, with a defined treatment plan.4
Treatment schedule
Patients received a dose of 3 mg/kg of melphalan using CHEMOSAT delivery system every 6 to 8 weeks for up to 6 treatment cycles.4,a
a Based on ideal body weight (IBW; maximum total dose, 220 mg)

More Than 1/3 of Patients Responded to Treatment With CHEMOSAT4
ORR reported in FOCUS was 36.3% (n = 33)4
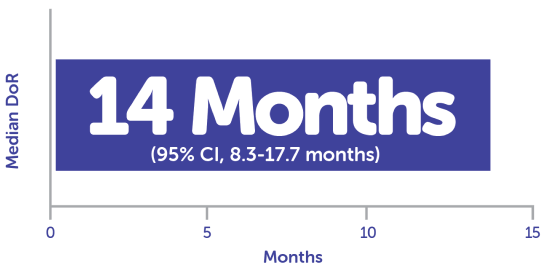
Median DoR in responders (n = 33) was 14 months4,c
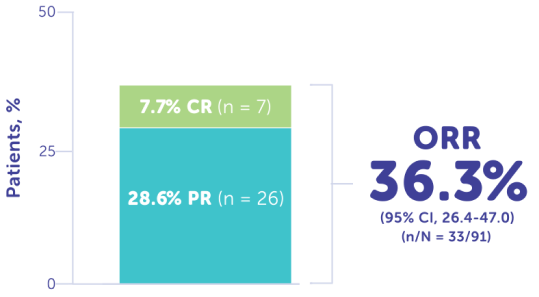
CR: Disappearance of all target lesionsa
PR: ≥30% Decrease in the sum of the long axis diameter of tumor target lesionsb
aAny pathological lymph nodes (target or nontarget) must be <10 mm in short-axis diameter. For nontarget lesions, disappearance of all nontarget lesions and normalization of tumor marker level.5
bUsing the baseline sum as reference.5
cCalculated using Kaplan-Meier method.4
Safety: Demonstrated
Safety & Tolerability4
Most common adverse events (>30% of patients)
• Nausea • Fatigue • Musculoskeletal pain
• Abdominal pain • Vomiting
All adverse events observed at a frequency of >10% in patients
| All grades, % | Grades 3 or 4, % | |
|---|---|---|
| Gastrointestinal disorders | ||
| Nausea | 57 | 0 |
| Abdominal paina | 39 | 1 |
| Vomitinga | 35 | 0 |
| Diarrheaa | 17 | 1 |
| General disorders | ||
| Fatiguea | 65 | 0 |
| Pyrexiaa | 16 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal paina | 46 | 1 |
| Groin pain | 11 | 0 |
| Respiratory disorders | ||
| Dyspneaa | 23 | 2 |
| Cougha | 15 | 0 |
| Nervous system disorders | ||
| Headachea | 19 | 0 |
| Lethargy | 12 | 0 |
| Dizzinessa | 11 | 0 |
| Injury and procedural complications | ||
| Contusion | 17 | 0 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 16 | 0 |
| Vascular disorders | ||
| Hemorrhagea | 15 | 1 |
| Hypotensiona | 13 | 3 |
aRepresents a composite of multiple, related preferred terms.
Serious adverse events occurred in 45% of patients who received treatment4
Serious adverse events occurring in ≥2% of patients were thrombocytopenia (10%), neutropenia (8%), febrile neutropenia (7%), platelet count decreased (6%), leukopenia (4.2%), cardiac arrest (3.2%), neutrophil count decreased (2.1%), hypoxia (2.1%), pleural effusion (2.1%), pulmonary edema (2.1%), and deep vein thrombosis (2.1%).4
Fatal adverse events occurred in 3 patients (3.2%) ; these included cardiac arrest, acute hepatic failure, and bacterial peritonitis and were deemed not to be related to treatment.4,6
Treatment was permanently discontinued because of adverse events in 18% of patients, with neutropenia being the most common reason (3.2%).4
Prospective & Retrospective Studies
Treatment naïve as well as previously treated patients have shown efficacy across various endpoints.
| N | Treatment Line | Patient Tumor Characteristics | ORR | OS | PFS | Safety | Reference | |
|---|---|---|---|---|---|---|---|---|
| Prospective Study | 35 | 60% treatment naïve |
No. of metastases ≥10 (57%) | 72% |
1 year OS - 77% 2 year OS - 43% mOS 19.1 months |
7.5 mPFS |
Majority developed grade 3/4 haematologic events 14 grade 3 non-haematologic events |
Meijer, T., et al 20207 |
| Retrospective Studies | 81 | 54% treatment naïve |
>10 lesions/>50% volume replacement (51.9%) | 60.5% | mOS 14.9 months | 8.4 mPFS |
Grade 3/4 events were observed in 27.7% of patients | Modi, S., et al 20228 |
| 51 | 43.1% treatment naïve |
Oligometastatic disease ≤ 3 deposits (23.5%)
>10 lesions/>50% volume replacement (31.4%) |
47% | mOS 15.3 months | 8.1 months |
37.5% grade 3-4 non-haematologic events | Karydis, I., et al 20171 | |
| 19 | 31% treatment naïve |
Not reported | 53% | mOS 16.7 months | 14.03 months |
2 cases grade 3a (coronary ischaemia)
1 case grade 3b (transfemoral bleeding with following surgery) |
Bruning, R., et al 20202 | |
| 16 | 25% treatment naïve |
Hepatic lesions ranged from 3 to >20
Median tumour load of 22.5% (interquartile range 10 to 25%) |
60% | mOS 27.4 months | 11.1 mPFS |
Grade 3/4 events were observed in 43.8% of patients | Artzner, C., et al 20199 |
ORR – overall response rate; PFS – progression free survival; mPFS – median progression free survival, OS – overall survival; mOS – median overall survival.